As far as I know right now, there are three ways to produce X-rays: High voltage spark discharge, Plasmoids, and Laser stimulation.
Starting off with High Voltage Spark Discharge
There are two processes by which x rays are produced in the anode of an x-ray tube. In one process, the deceleration of electrons produces x rays, and these x rays are called bremsstrahlung, or braking radiation.
This second process is atomic in nature and produces characteristic x-rays, so called because they are characteristic of the anode material.
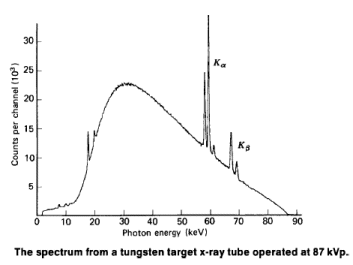
The x-ray spectrum in the figure above is typical of what is produced by an x-ray tube, showing a broad curve of bremsstrahlung radiation with characteristic x-ray peaks on it.
The smooth part of the spectrum is bremsstrahlung radiation, while the peaks are characteristic of the anode material. A different anode material would have characteristic x-ray peaks at different frequencies.
Each element has its own characteristic electromagnetic spectrum. This spectrum is defined by the orbitals of the electrons associated with the element.
X rays lie at the high-frequency end of an atom’s spectrum and are characteristic of the atom as well.
The spectrum in the figure above is collected over a period of time in which many electrons strike the anode, with a variety of possible outcomes for each hit. The broad range of x-ray energies in the bremsstrahlung radiation indicates that an incident electron’s energy is not usually converted entirely into photon energy. The highest-energy x-ray produced is one for which all of the electron’s energy was converted to photon energy. Thus the accelerating voltage and the maximum x-ray energy are related by conservation of energy. Electric potential energy is converted to kinetic energy and then to photon energy, so that a 100-kV accelerating voltage produces x-ray photons with a maximum energy of 100 keV.
See Bremsstrahlung
http://en.wikipedia.org/wiki/BremsstrahlungNext, some electrons excite atoms in the anode. Part of the energy that they deposit by collision with an atom results in one or more of the atom’s inner electrons being knocked into a higher orbit or the atom being ionized. When the anode’s atoms de-excite, they emit characteristic electromagnetic radiation. The most energetic of these are produced when an inner-shell vacancy is filled—that is, when an n=1 or n=2 shell electron has been excited to a higher level, and another electron falls into the vacant spot.
A characteristic x-ray is electromagnetic (EM) radiation emitted by an atom when an inner-shell vacancy is filled. X-rays created when an electron falls into an n=1 shell vacancy are called K type when they come from the next higher level; that is, an n=2 to n=1 transition.
The labels K, L, M,... come from the older alphabetical labeling of shells starting with K rather than using the principal quantum numbers 1, 2, 3, …. A more energetic K type x ray is produced when an electron falls into an n=1 shell vacancy from the n=3 shell; that is, an n=3 to n=1 transition. Similarly, when an electron falls into the n=2 shell from the n=3 shell, an L type x ray is created.
The energies of these x-rays depend on the energies of electron states in the particular atom and, thus, are characteristic of that element: every element has its own set of x-ray energies (see figure below). This property can be used to identify elements, for example, to find trace (small) amounts of an element in an environmental or biological sample.

When resonance is mentioned, in terms of absorption of x-rays, it means that the x-ray frequency matches one of the energy levels of the electron states in the particular atom.
High voltage spark discharge is not my favored method of x-ray generation because it has tradeoffs, limitations and engineering considerations.
For example, plasma facing is the biggest problem associated with ware resistance of the electrodes. The anode is particularly susceptible to it. The expanding plasma from the spark will do far more damage than the x-ray pulse. From Rout et al on anode materials in IEEE Transactions on Plasma Science.
Plasma sheath (pinch) current in a low energy (2.2 kJ) plasma focus device was measured fur different anode and insulator materials. Among the anode materials, the highest sheath current was observed with tungsten and the lowest with aluminum. Among the ceramic insulators the maximum plasma sheath current was obtained with quartz and the minimum with alumina. The computed high Z (atomic number) impurities in plasma sheath, however, were least in the plasma focus with alumina insulator. None of the nonceramic (plastic) insulators produced neutrons, as the plasma sheath was nonuniform and was highly contaminated with impurities
To generate high powered ions, we need very high energy x-rays. To do this, we need very high input voltage ate the electrodes.
In this case, the x-ray spectrum at the high end of the x-ray spectrum will be a bremsstrahlung spectrum with an endpoint near the maximum spark voltage.
But for a very high voltage of roughly 750 keV, most of the Bremsstrahlung spectrum will be concentrated at 1/3 of the endpoint or 250 keV.
Time to compare electrode materials based on what is known from other systems:
Tungsten is dense. Its melting point is 3422 C (Wikipedia). Electrical resistivity is 52.8 nOhm-m. Because of its high density, Tungsten is used as a high energy EMF shield material. It will absorb 99.9% of the x-rays at 250 keV in a 1 cm thick sheet (Numbers from NIST X-COM Data base).
Tungsten absorbs hydrogen but will re-emit it when hot (~100 C). There are no chemistry problems with noble gases.
A low Z element might make for a better electrode when producing x-rays. But because these materials are not dense, electrons will travel along way into them before they hit something.
I don’t know of any chemical reactions with boron, and it is very resistant to plasma facing. I would look at boron for electrodes.
A light electrode material is optimum when you are using it to produce a plasmoid. Note: Russ is now producing a plasmoid.
Because of its high density, Tungsten has a long history of being a robust electrode material in a number of plasma facing applications. It will produce more x-rays than any other common material but it can take the heat of the plasma and the chemistry. But it is very energy inefficient at converting spark energy to x-ray energy.
Carbon is dense. Carbon does not melt under most conditions but it sublimates (goes straight to gas) at 3642C. Electrical resistivity is 2500 nOhm-m. It will absorb 22% of the x-rays at 250 keV. Carbon forms a stable carbide with boron under plasma bombardment.
Carbon in the graphite phase (this includes nano-tubes and other carbon compounds) is very susceptible to etching by hydrogen plasma. This is how you can remove graphite from diamond in lab created diamonds. Sorry, diamond is one of the world’s best electrical insulators…
Carbon might be a good option is a noble gas environment.
Beryllium is not dense. Melts at 1290 C. Electrical resistivity is 36 nOhm-m. Beryllium will absorb only 17% of the x-rays at 250 keV. Comes with a warning from most vendors akin to ‘May cause death’. It is a known carcinogen as a dust or powder. It’s not like tobacco either. You get five years at best. Beryllium also has a nasty nuclear side. It will emit neutrons if photons of sufficient energy interact with it. So much for the radiation free system. With beryllium, x-rays become neutrons….
The melting point of the electrode is directly related to plasma facing tolerance. To vaporize the material you have to supply energy to melt it. Tungsten and carbon beat beryllium by a factor of three. Carbon is damaged by chemical reactions. But if you stay with noble gases, you might be OK. Carbon is a poor electrical conductor so it will absorb electrical energy needlessly. If carbon is doped with boron, its conductivity goes way up.
Boron doped carbon is my choice for the electrode material assuming plasmoid production which I favor.
If hydrogen is included in the gas mix, it might be fine in the boron-only-hydrogen environment.
Beryllium is energy efficient in terms of x-ray production, but Beryllium has a dark side in terms of radiation and health concerns.
The buckets electrodes filled with other material are a special case. I need to write another post on buckets because this post is getting too long.


